
HL Paper 3
Infrared (IR) spectroscopy is widely used as a technique in analytical chemistry.
The IR spectrum, mass spectrum and \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of an unknown compound, Y, of molecular formula \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}\) are as follows.
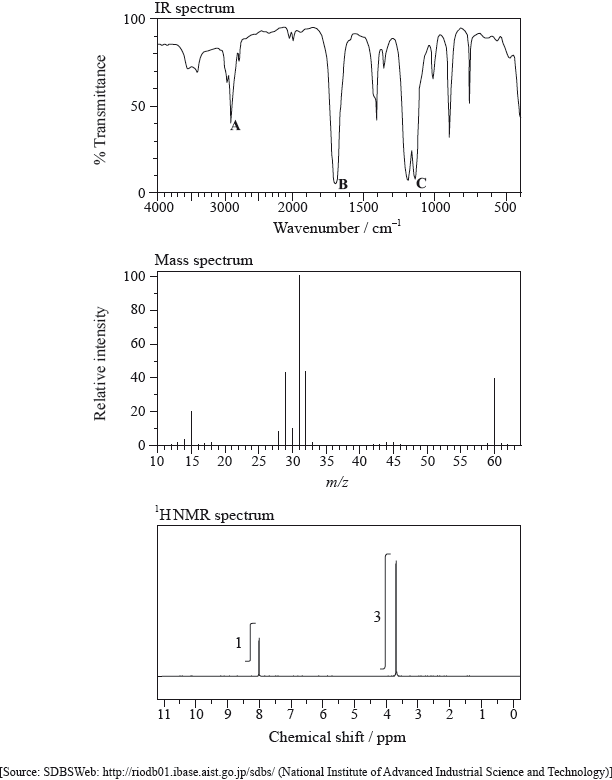
(i) Identify the bonds responsible for the peaks A, B and C in the IR spectrum of Y.
A:
B:
C:
(ii) In the mass spectrum of Y, deduce which ions the m/z values at 31 and 29 correspond to.
m/z = 31:
m/z = 29:
(iii) Identify the peaks at 3.76 and 8.07 ppm in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum.
3.76 ppm:
8.07 ppm:
(iv) State what information can be obtained from the integration trace about the hydrogen atoms responsible for the peak at 3.76 ppm in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum.
(v) Deduce the structure of Y.
(vi) Explain why tetramethylsilane (TMS) is suitable as a reference standard.
Markscheme
(i) A: C–H
B: C=O
C: C–O
Award [2] for three correct, [1] for two correct.
(ii) m/z = 31: \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{O}}^ + }\);
m/z = 29: \({\text{HC}}{{\text{O}}^ + }\);
Penalize missing + charge once only.
Elements can be given in any order (i.e. OCH3+, COH+, CHO+).
(iii) 3.76 ppm: \({\text{C}}{{\text{H}}_3}{\text{O}}\) and 8.07 ppm: HCO;
Allow CH3 for CH3O.
Allow RCOOCH2 and RCOOCH3 for 3.76 ppm and RCHO for 8.07 ppm.
(iv) three hydrogens in same environment;
Allow three times as many hydrogens in this environment as for the other peak.
(v) \({\text{HC}}{{\text{O}}_2}{\text{C}}{{\text{H}}_3}{\text{ / HCOOC}}{{\text{H}}_3}\);
(vi) all (12) protons/hydrogens in same chemical environment (and hence gives 1 peak);
absorbs upfield/away from most other protons/H’s;
low boiling point/bp / volatile / easily removed from sample;
not toxic;
highly unreactive (and hence does not interfere with sample) / inert;
Examiners report
In (c) (i), most candidates were able to relate B to C=O and C to C-O, but many gave O-H for A instead of C-H. In (ii), the most common mistake was candidates omitting the + charge. In (iii), only the best candidates scored the mark. One respondent stated in a G2 form that the value of 8.07 ppm is outside the range 9.4 to 10.0 ppm given in Table 18 of the Data Booklet and that candidates are not required to know how added shielding or deshielding from neighbouring groups affects the chemical shift. This is an interesting point and it should be emphasised that the spectra used are based on real spectra and, as is pointed out clearly in Table 18, chemical shift values may vary in different solvents and conditions. This is a very important point that teachers should emphasise to candidates in the teaching programme. In this question, candidates use a combination of spectra to deduce the structure of \({\text{HCOOC}}{{\text{H}}_{\text{3}}}\). In part (ii), \({\text{HC}}{{\text{O}}^ + }\) is identified corresponding to m/z = 29. Question part (iv) was usually well done but many candidates were not able to deduce the correct structure, \({\text{HCOOC}}{{\text{H}}_{\text{3}}}\) in (v). Many candidates gave the answer as ethanoic acid. Part (vi) was usually well answered and a significant number of candidates scored both marks.
Another structural isomer of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) is 2-bromo-2-methylpropane. Deduce the number of peaks and the splitting pattern in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of this isomer.
Number of peaks:
Splitting pattern:
Markscheme
Number of peaks:
1;
Splitting pattern:
singlet / it is not split;
Examiners report
The great majority scored full marks in (b).
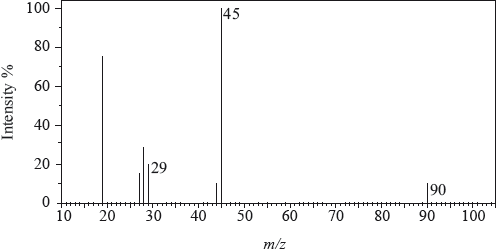
The mass spectrum of an unknown acidic compound, X, with empirical formula \({\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}\), is shown below.
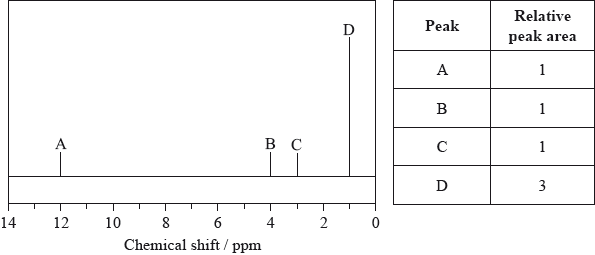
The low-resolution \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X shows four peaks. A simplified representation is shown alongside a table with relative peak areas.
Determine the relative molecular mass, to the nearest integer, of the compound from the mass spectrum and deduce the formula of the molecular ion.
Deduce the formula of the fragment responsible for the peak at 45.
Deduce the formula of the fragment responsible for the peak at 29.
Identify the group responsible for the peak at D.
Suggest a possible structure for X.
Peak B shows the following splitting pattern in the high-resolution spectrum.
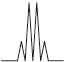
Explain the splitting pattern, indicating the hydrogen responsible for peak B.
Markscheme
90;
\({{\text{C}}_3}{{\text{H}}_6}{\text{O}}_3^ + \);
Penalize missing positive charge of ion only once in (a).
\({\text{COO}}{{\text{H}}^ + }\);
Accept C2H5O+.
Penalize missing positive charge of ion only once in (a).
\({\text{CH}}{{\text{O}}^ + }/{\text{CO}}{{\text{H}}^ + }{\text{ }}\);
Accept C2H5+/CH3CH2+.
Penalize missing positive charge of ion only once in (a).
\({\text{C}}{{\text{H}}_3}\)/methyl;
\({\text{C}}{{\text{H}}_3}{\text{CH(OH)COOH}}\);
Allow full or condensed structural formula.
quartet means next C has 3 H atoms / is \({\text{C}}{{\text{H}}_3}\);
due to the CH group;
due to relative orientation of spinning nuclei/protons;
with relative probabilities of 1,3,3,1;
OH group results in no splitting (due to rapid proton exchange);
Examiners report
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.
Compound X has the molecular formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{3}}}\) and is found in human perspiration.
Its infrared (IR) spectrum is represented below.
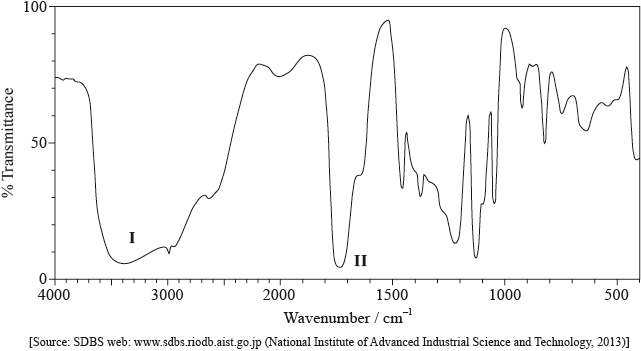
Deduce the bonds responsible for the absorptions labelled I and II.
I:
II:
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum recorded showed four peaks with the following chemical shift values (in ppm):
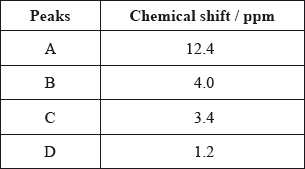
The integration trace for A:B:C:D was found to be 1:1:1:3.
Deduce what information can be obtained about the hydrogen atoms responsible for peak D at 1.2 ppm from the integration trace in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X.
Deduce the fragments in the mass spectrum which correspond to the following \(m{\text{/}}z\) values.
\(m{\text{/}}z = 45\):
\(m{\text{/}}z = 17\):
\(m{\text{/}}z = 15\):
Deduce the structural formula of X.
Y is an isomer of X, which contains the same functional groups. Deduce the structural formula of Y.
(i) Like X, 3-methylbutanoic acid is also a source of body odour. Deduce the \(m{\text{/}}z\) value for the molecular ion peak on the mass spectrum of this compound.
(ii) Ethyl propanoate (ethyl propionate) is an isomer of 3-methylbutanoic acid. Its \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum consists of four peaks.
Deduce the ratios of the areas under each peak in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of ethyl propanoate. For each peak, deduce the range of chemical shift values (in ppm), using Table 18 of the Data Booklet, and predict the splitting pattern.
Markscheme
I: O–H and II: C=O;
Do not allow CO for C=O.
Allow OH for O–H.
three hydrogens in same (chemical) environment / CH3/methyl (group);
Award [2] for all three correct, [1] for any two correct.
\(m{\text{/}}z = 45\):
\({\text{COO}}{{\text{H}}^ + }/{\text{C}}{{\text{O}}_2}{{\text{H}}^ + }/{{\text{C}}_2}{{\text{H}}_5}{{\text{O}}^ + }\);
\(m{\text{/}}z = 17\):
\({\text{O}}{{\text{H}}^ + }\);
\(m{\text{/}}z = 15\):
\({\text{CH}}_3^ + \);
Penalize missing + once only.
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)COOH}}/{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{O}}_{\text{2}}}{\text{H}}\);
Allow full or condensed structural formula.
\({\text{C}}{{\text{H}}_2}{\text{(OH)C}}{{\text{H}}_2}{\text{COOH}}/{\text{HO(C}}{{\text{H}}_2}{{\text{)}}_2}{\text{COH}}\);
Allow full or condensed structural formula.
(i) 102;
(ii) 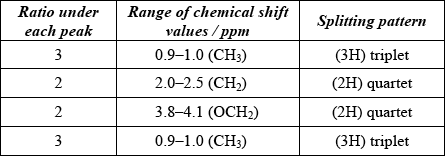
Award [3 max] for four correct rows.
Award [2 max] for any two or three correct rows and [1 max] for any correct row.
Examiners report
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.
The mass spectrum and infrared (IR) spectrum of a compound are shown below.
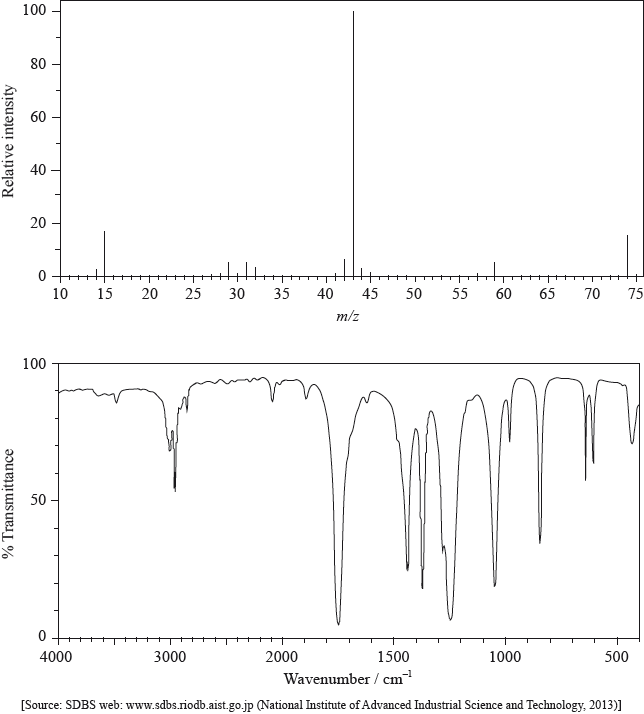
(i) State the information about this particular compound that can be derived from the mass spectrum and outline how it is found.
(ii) Suggest how the fragment with m/z = 43 is formed from the original molecule.
(i) Use the IR spectrum in the region 1600 – 1800 \({\text{c}}{{\text{m}}^{ - 1}}\) to deduce one functional group that is present in the compound and one group that is absent.
Present:
Absent:
(ii) The molecular formula of the compound is \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\). Explain, with reference to another region of the IR spectrum, why the compound could not be propanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\).
(iii) Deduce the structures of two possible isomers of propanoic acid consistent with the IR spectrum.
\(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectroscopy is often very useful in distinguishing between closely related compounds such as the ones shown below.
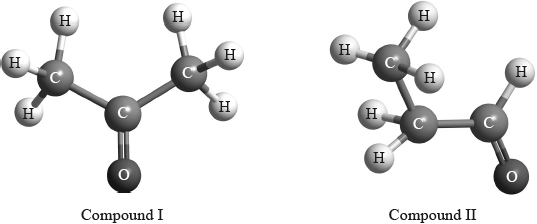
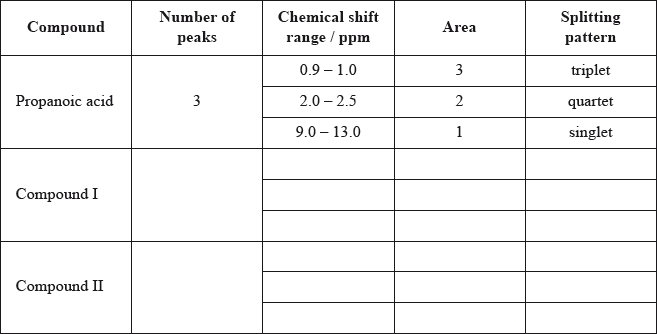
Deduce the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra you would expect for each compound and complete the table below. (The answers for propanoic acid are given as a guide.)
Note that some of the boxes may be blank.
Markscheme
(i) molar/molecular mass/\(M = 74{\text{ (g mo}}{{\text{l}}^{ - 1}}{\text{)}}\) / relative molecular mass/\({M_r} = 74\);
peak with highest m/z (ignoring any peak attributable to \(^{{\text{13}}}{\text{C}}\)) / found from parent/molecular ion peak;
Allow mass for m/z.
OR
compound has methyl/\({\text{C}}{{\text{H}}_{\text{3}}}\);
m/z = 15 due to \({\text{CH}}_3^ + \);
OR
compound has propyl/\({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}\)/isopropyl/\({\text{CH(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\)/acetyl/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}\);
m/z = 43 due to \({{\text{C}}_{\text{3}}}{\text{H}}_7^ + {\text{/CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}_2^ + {\text{/C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}^ + }\);
OR
compound has acetoxy/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}\);
m/z = 59 due to \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}{{\text{O}}^ + }\);
Fragment must contain + sign in relevant marks above.
Penalize missing charges where relevant once only in (a)(i) and (ii).
(ii) loss of \({\text{C}}{{\text{H}}_3}\)–O / loss of radical with m/z = 31 / formation of \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{{\text{O}}^ + }/{{\text{C}}_{\text{3}}}{\text{H}}_7^ + /{\text{CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}_2^ + \);
Penalize missing charges where relevant once only in (a)(i) and (ii).
(i) Present: carbonyl/C=O;
Do not accept aldehyde / ketone.
Accept ester/alkanoate only if m/z = 59 given in (a)(i).
Absent: carbon–carbon double bond/C=C/alkene;
(ii) no (broad) absorption at 2500 – 3300 \({\text{(c}}{{\text{m}}^{ - 1}}{\text{)}}\);
no O–H bond;
Award [1 max] for just stating “absorption at 1050-1410 (cm–1) / C–O bond present of alcohol/ester/ether”.
Do not accept just “C–O bond present”.
Accept “peak” for absorption.
(iii) Any two structures from:
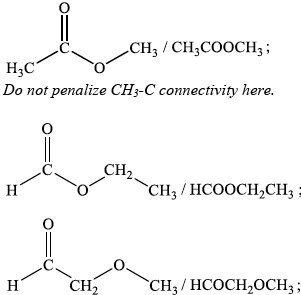
Award [1] for each 3 correct answers.
Award [5] for 13–14 correct answers.
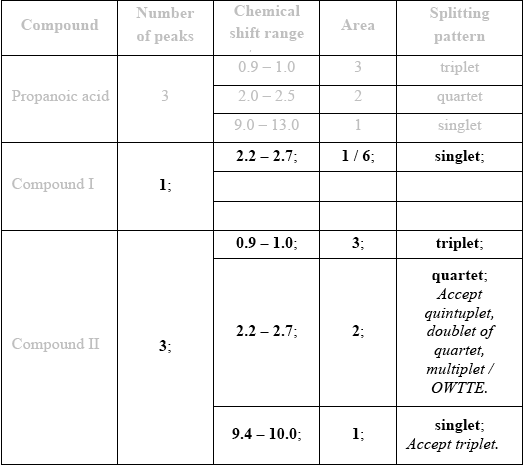
If more than one peak indicated for Compound I, then no “points”. Begin marking at Compound II and [3 max].
Examiners report
In general, most were able to deduce the molecular mass of the compound and how it is found. Unfortunately, some gave an account of the mechanism of fragmentation rather than giving information about this particular compound. Part (a) (i) asks about the mass spectrum; some candidates made the mistake of mentioning the IR spectrum at this point. It was encouraging to see the + sign normally included in any discussion of fragments in (a) (ii). The functional groups present and absent were usually identified correctly and most deduced why the compound could not be propanoic acid. Although, we allowed candidates to say that there was no OH bond present, they should be more careful to specify that it is the bond between the O and the H atoms that is not present. Care still needs to be taken in drawing structures, as in (b) (iii), to ensure the correct bond linkage. Many gave structures with an –OH group even though it had been ruled out in the previous part. Most were able to score well on the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) question although there were some inaccuracies in the chemical shifts suggested.
In general, most were able to deduce the molecular mass of the compound and how it is found. Unfortunately, some gave an account of the mechanism of fragmentation rather than giving information about this particular compound. Part (a) (i) asks about the mass spectrum; some candidates made the mistake of mentioning the IR spectrum at this point. It was encouraging to see the + sign normally included in any discussion of fragments in (a) (ii). The functional groups present and absent were usually identified correctly and most deduced why the compound could not be propanoic acid. Although, we allowed candidates to say that there was no OH bond present, they should be more careful to specify that it is the bond between the O and the H atoms that is not present. Care still needs to be taken in drawing structures, as in (b) (iii), to ensure the correct bond linkage. Many gave structures with an –OH group even though it had been ruled out in the previous part. Most were able to score well on the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) question although there were some inaccuracies in the chemical shifts suggested.
In general, most were able to deduce the molecular mass of the compound and how it is found. Unfortunately, some gave an account of the mechanism of fragmentation rather than giving information about this particular compound. Part (a) (i) asks about the mass spectrum; some candidates made the mistake of mentioning the IR spectrum at this point. It was encouraging to see the + sign normally included in any discussion of fragments in (a) (ii). The functional groups present and absent were usually identified correctly and most deduced why the compound could not be propanoic acid. Although, we allowed candidates to say that there was no OH bond present, they should be more careful to specify that it is the bond between the O and the H atoms that is not present. Care still needs to be taken in drawing structures, as in (b) (iii), to ensure the correct bond linkage. Many gave structures with an –OH group even though it had been ruled out in the previous part. Most were able to score well on the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) question although there were some inaccuracies in the chemical shifts suggested.
Typical proton chemical shift values are given in Table 18 of the Data Booklet. The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X contains three peaks. Details of two of these are shown in the table below.
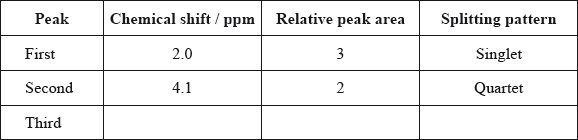
Deduce a possible structure for X that is consistent with the mass, IR and \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra.
Complete the table above by suggesting the chemical shift of the third peak, and state its relative peak area and splitting pattern.
Explain the splitting pattern of the peak at chemical shift 4.1 ppm.
Markscheme
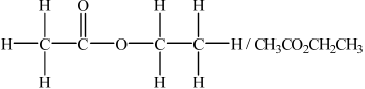 ;
;
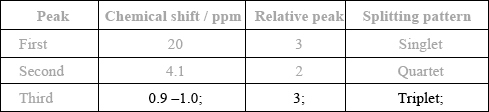
ECF from structure in (c)(i).
(quartet means) neighbouring C;
has 3 H atoms/protons;
Award [1] for stating CH3CH2.
Award [2] for stating CH3CH2 group and indicating number of protons.
Examiners report
In part (c), most of the better candidates deduced the structure as CH3COOCH2CH3 and subsequently the correct chemical shift, relative peak area and splitting patter for the third peak.
In part (c), most of the better candidates deduced the structure as CH3COOCH2CH3 and subsequently the correct chemical shift, relative peak area and splitting patter for the third peak.
The splitting pattern of the peak at 4.1 ppm was usually well answered. Many students displayed very good knowledge of \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra in this question.
The structure of an unknown compound A with empirical formula \({\text{C}}{{\text{H}}_{\text{2}}}\) can be determined using information from a variety of analytical techniques.
The infrared (IR) spectrum of A is shown below.
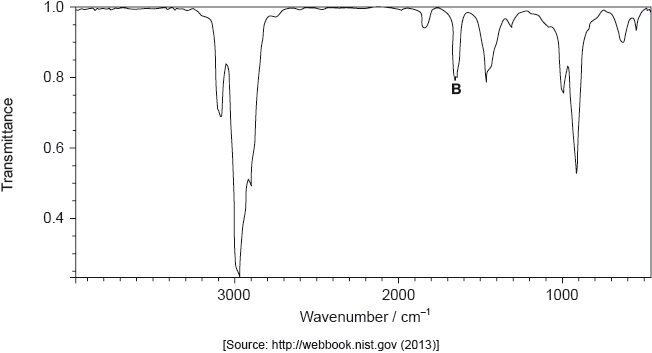
The mass spectrum of A is shown below.
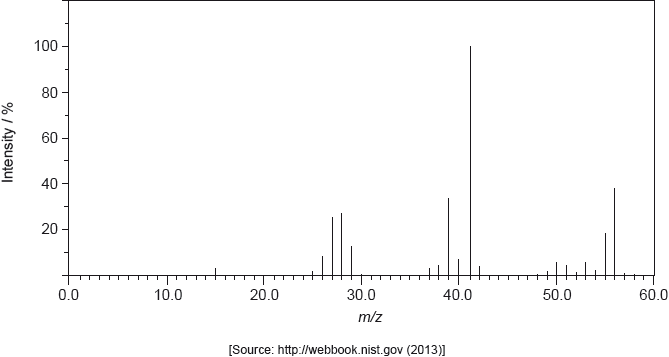
Deduce the formula of the molecular ion from the mass spectrum.
Explain the presence of a doublet in the high-resolution proton nuclear magnetic resonance (1H NMR) spectrum of A.
One isomer of A has only one signal in its \(^{\text{1}}{\text{H}}\) NMR spectrum. Deduce the structural formula of this isomer.
Markscheme
\({{\text{C}}_4}{\text{H}}_8^ + \);
Penalize missing charge only once in (i) and (ii).
produced by \({\text{(Hs in)}} = {\text{C}}{{\text{H}}_2}\) group;
adjacent C has 1 H atom;
\(n + 1\);
due to relative/(two) different orientations/alignment of spin of nuclei/protons/hydrogens (with applied/external magnetic field);
 ;
;
Accept full or condensed structural formula.
Examiners report
This question focused on some of the fundamental spectroscopic techniques (MS, IR and \(^{\text{1}}{\text{H}}\) NMR) used in analytical chemistry. The better candidates did well on this question though few scored full marks. In (a), the most common mistake was omission of the positive charge in (i). One G2 comment stated that isotopic effects in mass spectra with regard to the determination of the molecular ion peak would confuse students. This generally was not the case and although most got the \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\) formula a large majority of candidates simply did not read the question correctly which specifically asked for the formula of the molecular ion. In (ii) the correct formulas of the fragments were usually given. In (b), C=C was usually cited as the correct structural formula in (ii). The weaker candidates struggled with explaining the doublet in (iii). Cyclobutane was obtained by a large number of candidates in (iv). In (c) (i), the better candidates scored all three marks. In (ii), an understanding of the fingerprint region was poorly conveyed. There were two parts to this question – an outline of what happens on a molecular level when radiation in the fingerprint region is absorbed and how this region is used in chemical analysis. One G2 comment referred to the fact that the fingerprint region is not explicitly mentioned in the guide. Although this is true per se AS 3.2 does require a description of how the information from an IR spectrum can be used to identify bonds, and it would be assumed that the fingerprint region would be discussed in the context of teaching IR spectroscopy as part of the IB chemistry programme.
This question focused on some of the fundamental spectroscopic techniques (MS, IR and \(^{\text{1}}{\text{H}}\) NMR) used in analytical chemistry. The better candidates did well on this question though few scored full marks. In (a), the most common mistake was omission of the positive charge in (i). One G2 comment stated that isotopic effects in mass spectra with regard to the determination of the molecular ion peak would confuse students. This generally was not the case and although most got the \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\) formula a large majority of candidates simply did not read the question correctly which specifically asked for the formula of the molecular ion. In (ii) the correct formulas of the fragments were usually given. In (b), C=C was usually cited as the correct structural formula in (ii). The weaker candidates struggled with explaining the doublet in (iii). Cyclobutane was obtained by a large number of candidates in (iv). In (c) (i), the better candidates scored all three marks. In (ii), an understanding of the fingerprint region was poorly conveyed. There were two parts to this question – an outline of what happens on a molecular level when radiation in the fingerprint region is absorbed and how this region is used in chemical analysis. One G2 comment referred to the fact that the fingerprint region is not explicitly mentioned in the guide. Although this is true per se AS 3.2 does require a description of how the information from an IR spectrum can be used to identify bonds, and it would be assumed that the fingerprint region would be discussed in the context of teaching IR spectroscopy as part of the IB chemistry programme.
This question focused on some of the fundamental spectroscopic techniques (MS, IR and \(^{\text{1}}{\text{H}}\) NMR) used in analytical chemistry. The better candidates did well on this question though few scored full marks. In (a), the most common mistake was omission of the positive charge in (i). One G2 comment stated that isotopic effects in mass spectra with regard to the determination of the molecular ion peak would confuse students. This generally was not the case and although most got the \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\) formula a large majority of candidates simply did not read the question correctly which specifically asked for the formula of the molecular ion. In (ii) the correct formulas of the fragments were usually given. In (b), C=C was usually cited as the correct structural formula in (ii). The weaker candidates struggled with explaining the doublet in (iii). Cyclobutane was obtained by a large number of candidates in (iv). In (c) (i), the better candidates scored all three marks. In (ii), an understanding of the fingerprint region was poorly conveyed. There were two parts to this question – an outline of what happens on a molecular level when radiation in the fingerprint region is absorbed and how this region is used in chemical analysis. One G2 comment referred to the fact that the fingerprint region is not explicitly mentioned in the guide. Although this is true per se AS 3.2 does require a description of how the information from an IR spectrum can be used to identify bonds, and it would be assumed that the fingerprint region would be discussed in the context of teaching IR spectroscopy as part of the IB chemistry programme.
Biological pigments include a variety of chemical structures with diverse functions.
The graph shows the conversion of hemoglobin to oxyhemoglobin.
Hb(aq) + 4O2(g) \( \rightleftharpoons \) Hb(O2)4(aq)
The partial pressure of oxygen gas, p(O2) is proportional to its concentration.
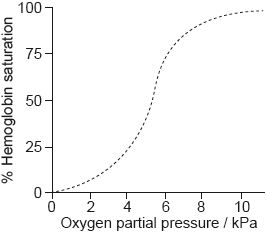
Explain the shape of the curve at low oxygen partial pressure up to about 5 kPa.
Sketch a graph on the axes above to show the effect of decreasing pH on the binding of oxygen to hemoglobin (the Bohr Effect).
Outline the effect of decreasing pH on the oxygen saturation of hemoglobin.
Markscheme
binding of O2 «to one active site» affects shape of Hb/other active sites
OR
binding of one O2 «molecule» affects binding of other O2 «molecules»
increasing affinity of Hb to O2
OR
enhanced binding of «further» O2 «molecules»
OR
cooperative binding
[2 marks]
sketching right shift of curve on graph
[1 mark]
decreases «oxygen saturation»
Accept “hemoglobin binds to O2 with less affinity".
[1 mark]
Examiners report
Aspirin is one of the most widely used drugs in the world.
Aspirin was synthesized from 2.65 g of salicylic acid (2-hydroxybenzoic acid) (Mr = 138.13) and 2.51 g of ethanoic anhydride (Mr = 102.10).
Suggest two absorbances, other than the absorbances due to the ring structure and C–H bonds, that would be present in the infrared (IR) spectrum of aspirin.
State two techniques, other than IR spectroscopy, which could be used to confirm the identity of aspirin.
Markscheme
Any two of:
2500–3000 «cm–1» / «absorbance» due to O–H in carboxyl
1700–1750 «cm–1» / «absorbance» due to C=O in carboxyl/ethanoate
1050–1410 «cm–1» / «absorbance» due to C–O bond in carboxyl/ethanoate
Accept “carboxylic acid” for “carboxyl”, “acetate/ester” for “ethanoate”.
Accept specific wavenumber once within indicated range.
Do not award mark if reference is made to an alcohol/ether.
[2 marks]
Any two of:
melting point
mass spectrometry/MS
high-performance liquid chromatography/HPLC
NMR/nuclear magnetic resonance
X-ray crystallography
elemental analysis
Accept “spectroscopy” instead of “spectrometry” where mentioned but not “spectrum”.
Accept “ultraviolet «-visible» spectroscopy/UV/UV-Vis”.
Do not accept “gas chromatography/GC”.
Accept “thin-layer chromatography/TLC” as an alternative to “HPLC”.
[2 marks]
Examiners report
The molecule of an unknown straight-chain compound consists of 4 carbon, 8 hydrogen, and 1 oxygen atoms. The 1H NMR spectrum of the compound is given below (the numbers next to integration traces correspond to areas under each peak).
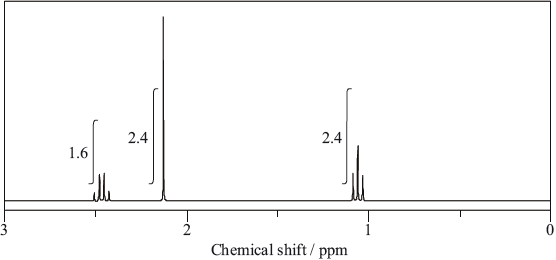
Calculate the number of hydrogen atoms for peaks with chemical shifts of 2.15 and 2.4–2.5 ppm. An example for the peak at 1.0–1.1 ppm is given.
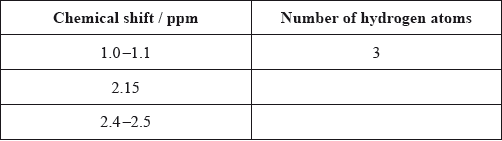
Analyse the splitting pattern of each peak and determine the relative positions of hydrogen atoms in the molecule. One example is given.
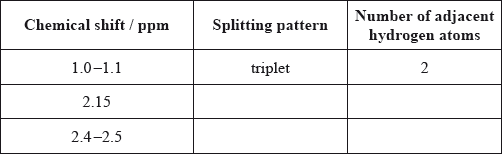
Using the information from (a) and (b), deduce the structural formula of the organic compound.
Markscheme
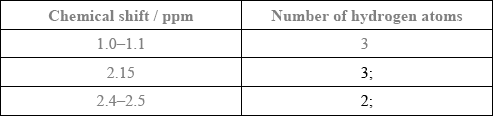
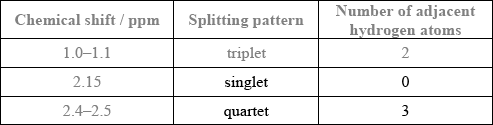
Award [1] for both splitting patterns correct.
Award [1] for both number of adjacent hydrogen atoms correct.
\({\text{C}}{{\text{H}}_3}{\text{COC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
Accept more detailed formula.
Examiners report
Option A proved to be very popular. Some candidates had difficulty explaining the purpose of the monochromator and some muddled Qualitative and Quantitative, but a reasonable proportion explained the latter. Many students were able to describe the practical method of column chromatography but were not able to explain the process in terms of adsorption, partition and retention. While many candidates knew about ‘d’ orbital splitting some forgot to explain the change in magnitude of the splitting, and a significant few thought that fewer ‘d’ electrons in the \({\text{C}}{{\text{r}}^{3 + }}\) ion would cause less repulsion and so less splitting.
Option A proved to be very popular. Some candidates had difficulty explaining the purpose of the monochromator and some muddled Qualitative and Quantitative, but a reasonable proportion explained the latter. Many students were able to describe the practical method of column chromatography but were not able to explain the process in terms of adsorption, partition and retention. While many candidates knew about ‘d’ orbital splitting some forgot to explain the change in magnitude of the splitting, and a significant few thought that fewer ‘d’ electrons in the \({\text{C}}{{\text{r}}^{3 + }}\) ion would cause less repulsion and so less splitting.
Option A proved to be very popular. Some candidates had difficulty explaining the purpose of the monochromator and some muddled Qualitative and Quantitative, but a reasonable proportion explained the latter. Many students were able to describe the practical method of column chromatography but were not able to explain the process in terms of adsorption, partition and retention. While many candidates knew about ‘d’ orbital splitting some forgot to explain the change in magnitude of the splitting, and a significant few thought that fewer ‘d’ electrons in the \({\text{C}}{{\text{r}}^{3 + }}\) ion would cause less repulsion and so less splitting.
The structures of morphine, diamorphine and codeine are given in section 37 of the data booklet.
Methadone is used to treat heroin addiction. 1H NMR spectroscopy can be used to study its structure.
Predict the number of different hydrogen environments in the molecule ignoring the benzene rings.
Predict the chemical shift and the splitting pattern seen for the hydrogens on the carbon atom circled in the diagram. Use section 27 of the data booklet.
Markscheme
6
[1 mark]
Chemical shift:
2.2–2.7 «ppm»
Splitting pattern:
quartet/q
[2 marks]
Examiners report
Ibuprofen and paracetamol are mild analgesics. One of the IR spectra below belongs to ibuprofen and the other to paracetamol. The structures of both compounds are given in section 37 of the data booklet.
Both spectra show a peak at wavenumber 1700 cm–1. Identify the bond responsible for this peak.
Deduce which spectrum belongs to paracetamol, giving two reasons for your choice. Use section 26 of the data booklet.
Describe how mild analgesics function.
Markscheme
C=O
Accept “carbonyl”.
X (must be identified) AND
Any two of:
For X:
N–H «absorption» AND at 3300 – 3500 «cm–1» ✔
O–H «absorption» in phenol AND at 3200 – 3600 «cm–1» ✔
absence of OH «absorption» in carboxylic acid AND 2500 – 3000 «cm–1»
Accept any specific wavenumber in the range 3300–3380 «cm–1» for M1.
Accept any specific wavenumber in the range 3100–3200 «cm–1».
Award [1 max] if Y is incorrectly identified for paracetamol but if a correct reason/reasons is/are given for the bond absorption(s).
[Max 2 Marks]
prevents/interferes with the production of prostaglandins
OR
prevents/interferes with the production of substances responsible for inflammation/pain/fever at the site of injury/source of pain
Examiners report
In recent years several antiviral medications have been produced. One of these medications is oseltamivir (Tamiflu).
Identify the functional group circled in the structure of oseltamivir.
Predict the number of signals and relative integration you would expect to see in the nuclear magnetic resonance spectroscopy (1H NMR) spectrum for the circled portion in the structure.
Number of signals:
Relative integration:
Oseltamivir is a chiral compound.
(i) Identify an apparatus that can be used to distinguish between its enantiomers.
(ii) Explain how the differentiation between the enantiomers is obtained using this apparatus.
Markscheme
ether
Do not accept “C-O-C”.
Number of signals: 3 «signals»
Relative integration: 6:4:1
Accept any correct ratio order.
(i)
polarimeter
Accept other alternative techniques such as “GC/HLPC/chromatography using a chiral column”.
Do not accept just “polarizer”.
(ii)
«plane-»polarized light passed through sample
analyzer/second polarizer determines the angle of rotation of the plane of polarized light
OR
each enantiomer will rotate plane «of plane-»polarized light in opposite directions «by the same angle»
Accept explanation related to other alternative techniques such as GC/ HLPC/chromatography using a chiral column.
Award [2] for “(+)/d rotates plane of polarization to the right AND (-)/l rotates plane of polarization to the left”.